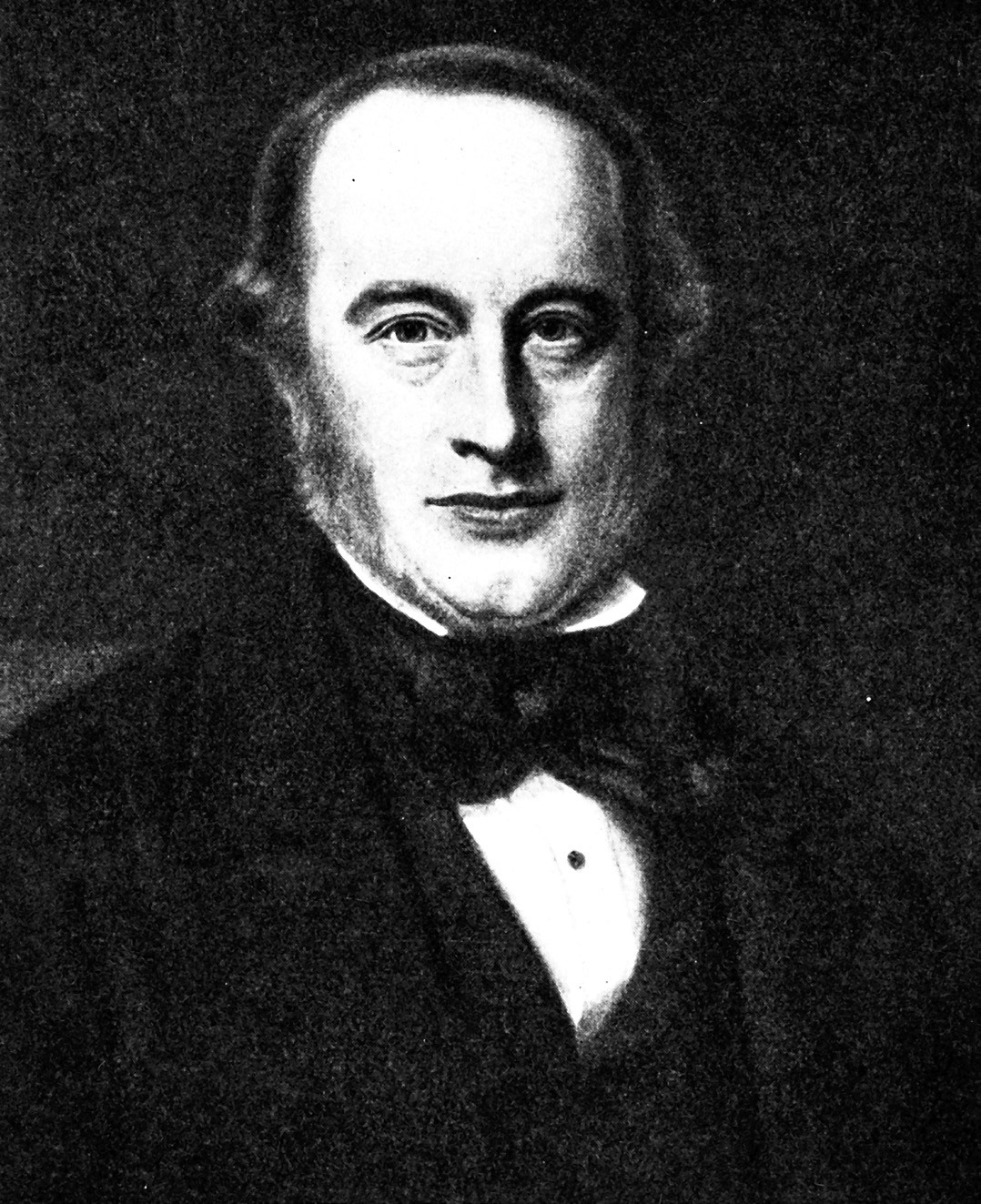
James Joule was a Manchester brewer and also a very keen experimenter. His careful measurements showed that the same amount of mechanical work always led to the same rise in temperature for a fixed mass of a substance (such as water). This became known as the ‘mechanical equivalence of heat’. He also showed that the heating effect of an electric current (I2× R× t) always resulted in the same rise in temperature. These discoveries were crucial in establishing the fundamental concept of energy.
It is said that Joule’s interest in physics was such that he took time out from his honeymoon in the Alps to measure the temperature of the water at the top and at the bottom of a waterfall, although there is no record of any results to support the stor y.
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




