The kaleidoscope of colours in a flower garden is due to the nature of the molecules in the petals. However, there is more to the chemistry of these floral displays than meets the eye.
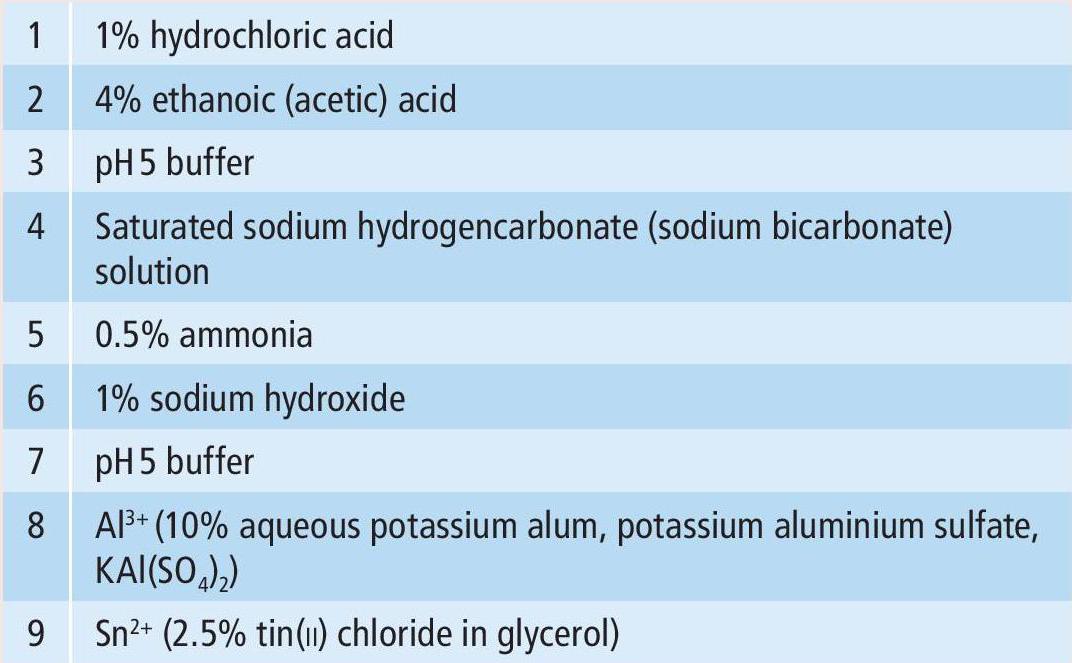
You may be familiar with the fact that extracts from red cabbage will change colour when exposed to solutions of different acidities and alkalinities, leading to the use of red cabbage as a pH indicator (see CHEMISTRY REVIEW Vol. 29, No. 3, 'Red cabbage indicators'). The molecules that give rise to this phenomenon are anthocyanins — flavonoid (phenolic) compounds, present as pigments in a range of plants. It is interesting to explore the colour spectrum that can be produced from flowers and other plant materials when their extracts are mixed with a variety of dilute solutions. ‘Lab page’ explains how you can carry out these experiments at home. Here we can just enjoy some of the results.
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




