Simon Cotton
Creating flavours in cheese
All cheese starts with the same basic ingredients, but clever chemistry conjures up a wealth of different flavours

EXAM LINKS
This article links to the following topics in the AQA, Edexcel, OCR, WJEC, CCEA, SQA and IB Diploma exam specifications:
■ amino acids
■ amines
■ carbonyl compounds, aldehydes and ketones
■ carboxylic acids
■ enzymes
■ esters
■ oxidation and reduction
Several thousand years ago, according to legend, a traveller set off on a long journey, taking some milk with them. They carried the milk in a pouch made from the stomach of a ruminant, such as a sheep. When, sometime later, they went to drink the milk, they found that it had turned into a white solid with a pleasant taste. They had hit upon the first long-life milk, which today we call cheese. The ruminant’s stomach lining contained rennet, which is a complex set of enzymes including chymosin (a protease enzyme) and other enzymes such as pepsin (another protease) and a lipase (which hydrolyses fats). The rennet was responsible for forming the first cheese, and it is still used today.
Cheese is a foodstuff rich in proteins and fat, providing us with essential amino acids as well as vitamins and minerals such as calcium. There are many types of cheese, each with its own flavour, and there is a rich chemistry that explains the origin of these differences.
Cheesemaking, cottage cheese and flavour
Most cheese is made from cow’s milk. This is usually pasteurised by brief heating, to kill off any dangerous pathogenic bacteria, and then cooled. At this stage rennet and starter bacteria (usually from the Streptococcaceae and Lactobacillaceae families) are added. The mixture is then digested at 30–40°C, when the starter bacteria ferment lactose (a sugar found in milk), to form lactic acid (2-hydroxypropanoic acid). This reduces the pH to a value around 4.6, when enzymes such as chymosin (also known as rennin) can coagulate the casein (the main protein in the milk), forming curds.
These curds are allowed to set for an hour or two before the liquid whey is separated from the curds. Cottage cheese is made from freshly drained cheese curds. It does not have much flavour to speak of, as there has not been enough time for a deep flavour to develop. Most cheese is stored to ripen.



Generating flavour
The three main types of substance in milk are protein (mainly casein), lipid (in milk fat) and sugar (lactose). Cheese develops a smell when these molecules are broken down into smaller ones by enzymes in the bacteria. Molecules with a smell have relative molecular masses of around 300 or less.
Different bacterial enzymes produce different flavoured cheeses. The starter bacteria mentioned above are the ones that also help hard cheeses, such as Cheddar, to ripen and generate flavour molecules by breaking down proteins. Other cheeses have different microorganisms added. Blue cheeses, for example Stilton, Gorgonzola and French blues such as Roquefort, are made by adding the blue-green Penicillium roqueforti mould, as well as the starter bacteria. After a few days of initial ripening, the cheeses are pierced to allow access of oxygen, which speeds up the process.
Surface-ripened cheese, like Camembert, are coated with Penicillium camemberti mould. They ripen from the outside inwards. Smear-ripened cheeses, such as Limburger and Brick, are also surface-ripened, this time by Brevibacterium linens bacteria on the surface.
Salt is added to cheeses at an early stage, partly as a flavouring but particularly to control the growth of microorganisms (by drawing water out of cells).
Lipids
Lipids are esters, so they are hydrolysed into their constituent parts, carboxylic acids and glycerol (propane-1,2,3-triol, an alcohol) in a process called lipolysis (Figure 1 and CHEMISTRY REVIEW Vol. 31, No. 3, pp. 27–29).
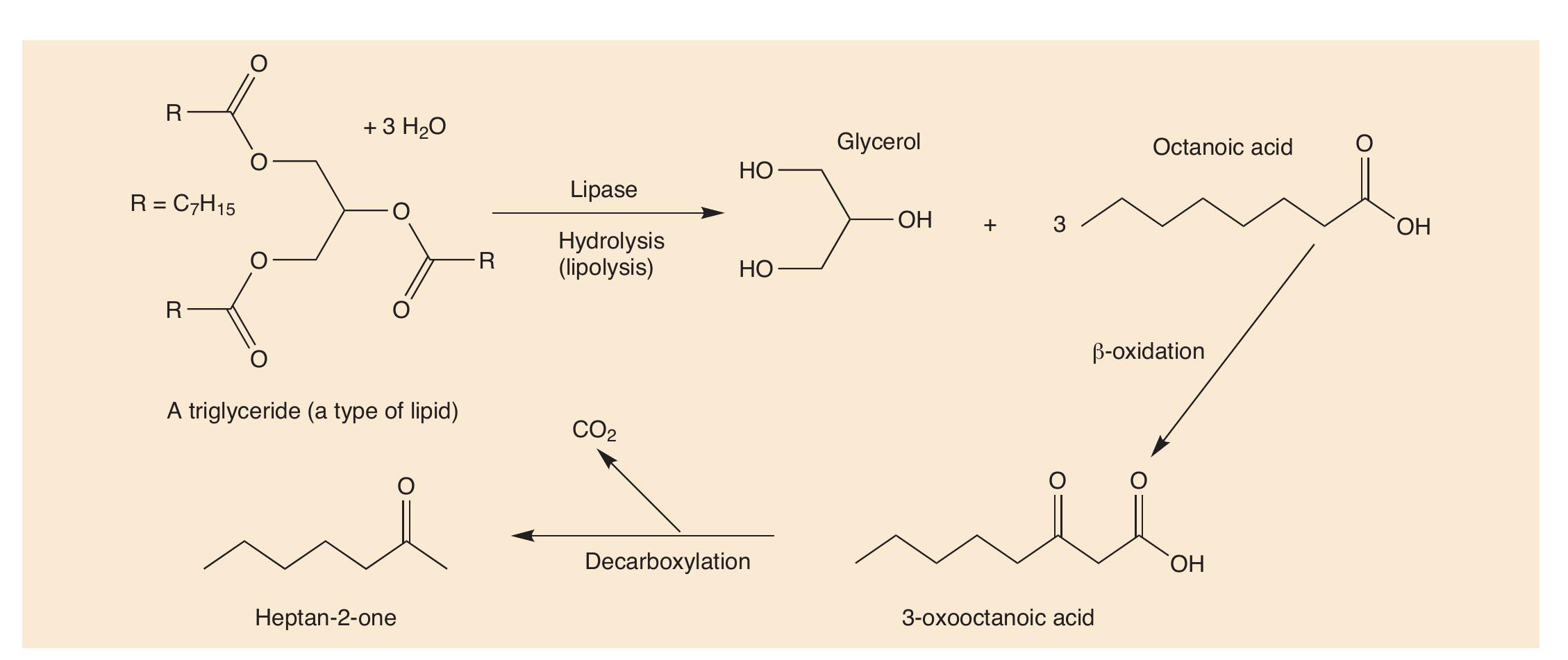
By themselves, carboxylic acids (CHEMISTRY REVIEW Vol 15, No. 1, pp. 28–31) do not have very wholesome odours (e.g. 3-methylbutanoic acid smells like old socks), but in the cheese this blends with the smells of other odorant molecules. More importantly, carboxylic acids are often themselves changed into other molecules, reacting with alcohols to form esters with stronger and more pleasant smells. Carboxylic acids are also broken up into the key odorants in blue cheeses.

Blue cheeses
The distinctive flavour in the famous blue cheeses, like Stilton, Roquefort and Gorgonzola, is caused by alkan-2-ones, often called methyl ketones, because they have a methyl group next to C=O. Heptan-2-one (C7H14O, Figure 1) and nonan-2-one (C9H18O) are the molecules mainly linked with blue-cheese smell.
Carboxylic acids are catabolised by enzymes in the Penicillum roqueforti bacterium. The acids are oxidised to β-hydroxyacids, then to b-ketoacids, which are decarboxylated to ketones that have one carbon atom fewer than the initial acid. ‘Natural’ carboxylic acids usually have an even number of carbon atoms, because they are biosynthesised from C2 units using acetyl-CoA (acetyl coenzyme A) and get turned into odd-carbon ketones when they lose a carbon dioxide molecule.
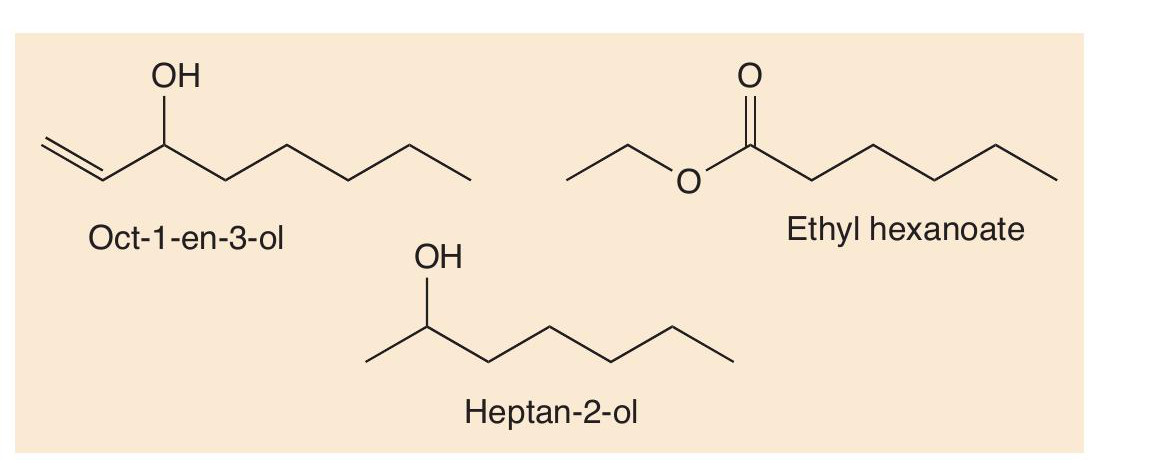
The R groups in the lipids usually have many more than 10 carbon atoms, so that they have to undergo multiple oxidations to make heptan-2-one and nonan-2-one. Heptan-2- one and nonan-2-one are the most important odorants in blue cheeses but are not the only ones. Others, including oct-1-en- 3-ol, heptan-2-ol and ethyl hexanoate, are also important in Gorgonzola (Figure 2), broadening out the smell of this cheese.
Lactose and Camembert
Lactose, or ‘milk sugar’, is an important source of flavouring — notably in surface mould-ripened cheeses, such as Camembert and Brie. Initially, lactose is split into glucose and galactose, both of which are further broken into lactic acid. The Penicillium camemberti mould, which the cheeses develop on their surface, decomposes lactic acid into carbon dioxide and water. This loss of acid raises the surface pH from around 4.6 to 7 and removes insoluble calcium phosphate from the centre, precipitating it on the surface and causing the centre of the cheese to soften as the casein micelles separate.

An interesting reaction involving lactate (a salt or ester of lactic acid) occurs in Gruyère and Emmental cheeses, which often contain holes or ‘eyes’. The initial lactose fermentation is followed by a secondary fermentation, due to the bacterium Propionibacterium freudenreichii, which converts lactate into ethanoate, propanoate and also gaseous carbon dioxide. The carbon dioxide migrates through the cheese, accumulating and forming holes in the cheese. The reaction can be summarised as:
3 lactate ➝ 2 propanoate + 1 ethanoate + 1 carbon dioxide
A wide range of flavour molecules is involved in Camembert. Other compounds derived from lactose include butane-2,3-dione (Figure 3), which contributes a buttery note, and ethanol. There are many other odorants associated with this cheese, for example ammonia, which is formed when amine (–NH2) groups are removed from amino acid molecules (i.e. deamination). Deamination occurs during the process known as proteolysis, which is the breakdown of proteins into smaller polypeptides or amino acids. The loss of ammonia through deamination is why you can sometimes smell ammonia on ripe Camembert.
Flavour does not just come from the molecules formed in the reactions outlined here. These molecules also react with other molecules, generating different flavours. For example, ethanol reacts with acids to form esters, which have stronger odours.
Proteolysis
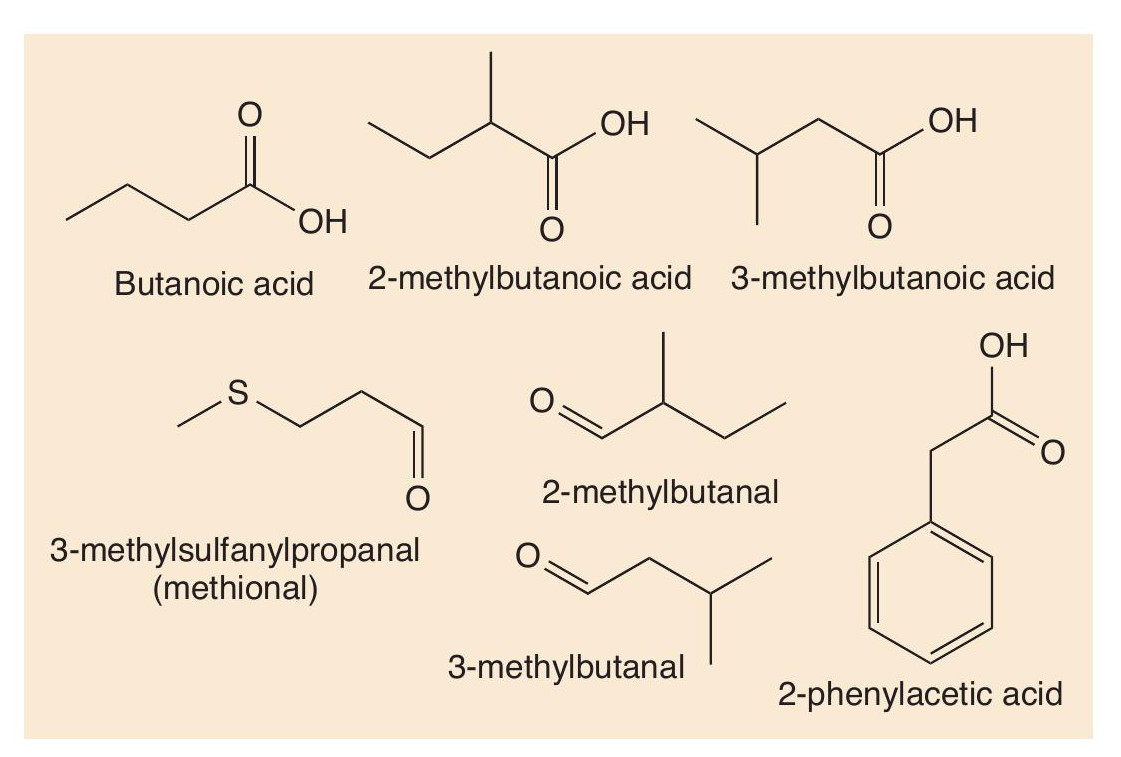
Proteins are made of long chains of amino acids linked together with peptide bonds. Proteolysis breaks down proteins, such as casein, first into peptides and then into amino acids. These amino acids contribute their taste to cheese, but more importantly undergo many reactions (decarboxylation, deamination, oxidation and reduction) that create more types of short-chain volatile molecule. Using valine as an example, Figure 4 shows some of the important odorant molecules that form.
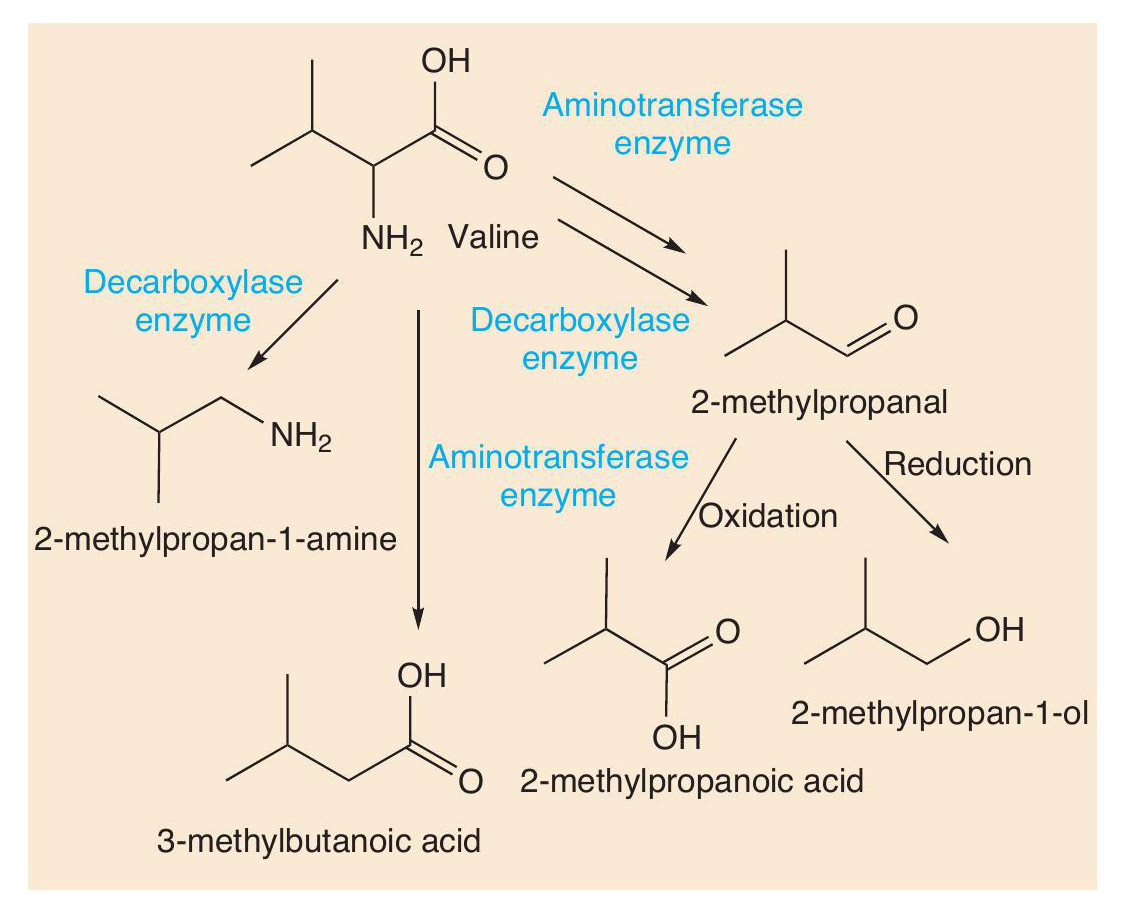
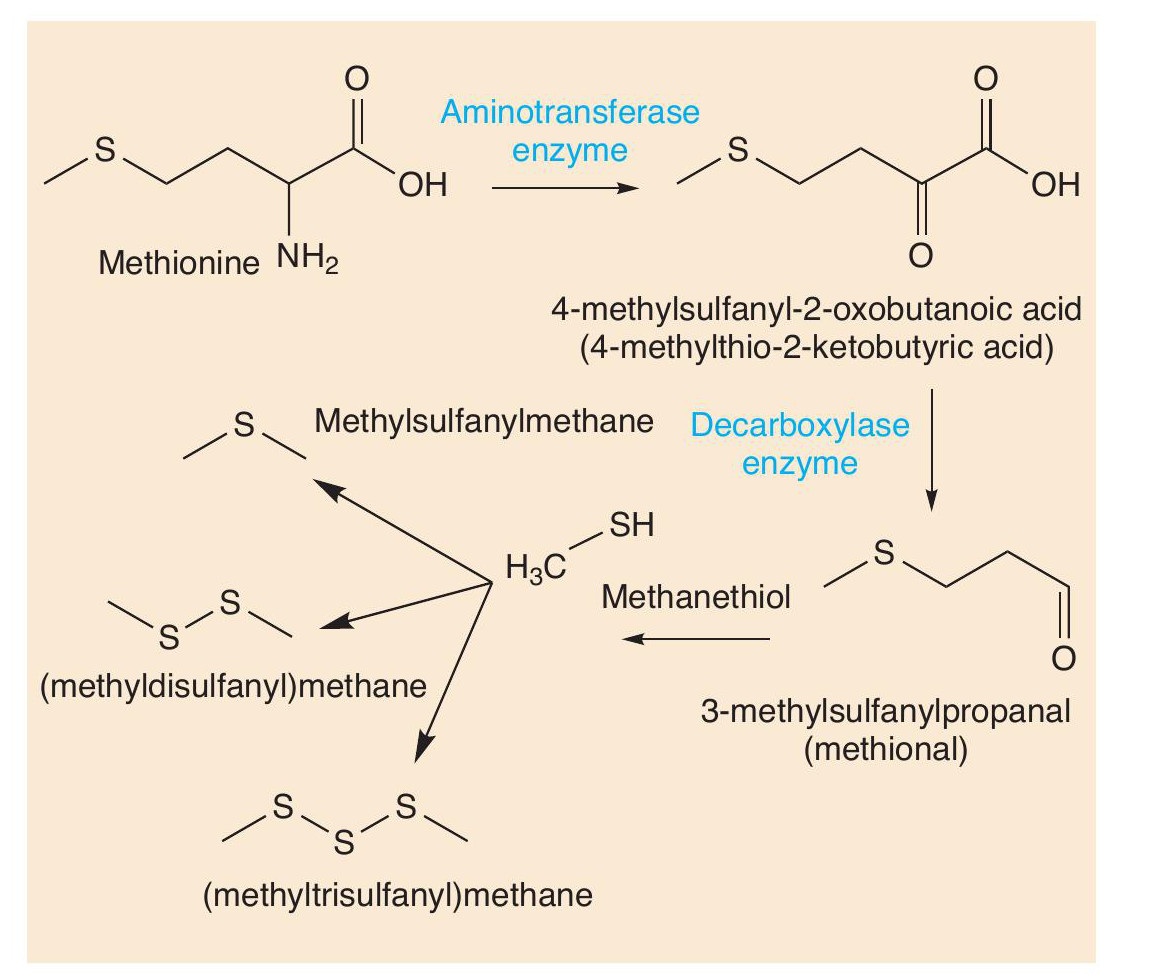
Such reactions also occur with other amino acids. Sulfur compounds are well-known for being smelly (see CHEMISTRY REVIEW Vol. 32, No. 2, p. 34). The sulfur-containing amino acid methionine is an important molecule in cheese. Two reactions change methionine into methional. Methional gives its smell to boiled potatoes, but along with other molecules contributes to the smell of Cheddar and Camembert. Methional also gets converted into methanethiol (CH3SH), which in turn is converted into other smelly sulfur compounds, like (CH3)2S, (CH3)2S2 and (CH 3)2S3 (Figure 5). There is one sulfur compound — the thioester CH3CH2C=O(SCH3) — that does indeed have a Camembert-like smell.
Some of the important odorants in Gruyère cheese are shown in Figure 6. Figure 7 shows how some of these molecules are formed from another amino acid, leucine. Malty and nutty notes are supplied to several cheeses by 3-methylbutanal, which is formed in cheese from leucine by catabolism. First the leucine undergoes enzyme-catalysed transamination into a keto acid, then another enzyme catalyses the decarboxylation of the acid, forming the aldehyde 3-methylbutanal. This aldehyde can be reduced to 3-methylbutan-1-ol and oxidised to 3-methylbutanoic acid.

Cheddar
Cheddar is one of the most popular cheeses in the world. Cheddar and other similar hard cheeses contain flavour molecules generated over several months by enzymes, mainly in the Lactobacilli that they contain. Unlike other cheeses (e.g. blues), which contain a unique ‘character-impact’ type molecule, Cheddar flavour comes from a balance of volatile molecules belonging to several families, including carboxylic acids, aldehydes and esters (Figure 8). The formation of several of these types of molecule was discussed earlier. If any one of them predominated, then the cheese would have a very disagreeable smell and taste. It is their blend and balance that make Cheddar agreeable.

Despite there only being three types of starting material from which they are derived, there is a rich variety of flavours among cheeses, attributable to the large number of volatile odorant molecules formed through the action of friendly microorganisms and the complex variety of reactions that occur.

PRACTICE EXAM QUESTIONS
1 Apply what you know about esterification to suggest two compounds that could be used to make the thioester CH3CH2C=O(SCH3). (2 marks)
2 Look at the structures of 3-methylbutanal, 3-methylbutan- 1-ol and 3-methylbutanoic acid in Figure 7. For each of the following pairs, suggest a reaction that could be carried out, which would give a positive result for the first one of the pair, but not for the second. (9 marks)
a 3-methylbutanal and 3-methylbutan-1-ol
b 3-methylbutan-1-ol and 3-methylbutanoic acid
c 3-methylbutanoic acid and 3-methylbutan-1-ol
GLOSSARY
Acetyl coenzyme A A molecule that is involved in many biological processes involving transfer of a two-carbon acetyl, CH3 CO, unit.
Amino acid In chemistry, an amino acid is any molecule that contains both amine (–NH2) and carboxyl (–COOH) functional groups. In biochemistry the term is used specifically to refer to alpha-amino acids, in which the amine and carboxylic acid functional groups are attached to the same carbon (called the alpha-carbon), which also bears a hydrogen atom and a distinctive chemical group called the R group. The R group is referred to as the side-chain and can vary in size, shape, electrical charge and hydrophobicity. Proteins are built from approximately 20 naturally occurring amino acids.
Catabolism A set of metabolic (biological) reactions that breaks down large molecules into smaller ones.
DecarboxylationA reaction in which a carboxyl group (–COOH) is lost from a carboxylic acid, resulting in the release of carbon dioxide (CO2).
Ester A chemical group (RCOOR) produced by a condensation reaction between an alcohol and a carboxylic acid.
Hydrolysis A reaction in which a molecule of water, or an ion derived from it (H+or OH–), decomposes another molecule by splitting it into two smaller ones.
Lipolysis The metabolic reaction in which a lipid (triglyceride) is hydrolysed to a molecule of glycerol and three carboxylic acids. It is a form of hydrolysis.
Micelles Small, spherical clusters of amphipathic molecules (i.e. they have both polar and non-polar ends) formed in an aqueous environment, as the non-polar (hydrophobic) parts of the molecules gather in the centre of the micelle, while the polar (hydrophilic) parts are on the outside, where they are solvated by water.
Peptide bond (amide bond)A covalent bond formed by the condensation of an amine group (–NH2) and a carboxyl group (–COOH) with the elimination of water. Amino acids are linked by peptide bonds within a protein.
Peptides Molecules made when two or more amino acids link together through peptide bonds. A long peptide molecule is called a polypeptide, which can fold up in a specific way to form a protein.
Protease Any enzyme that catalyses the hydrolysis (digestion) of proteins into smaller molecules (peptides and amino acids), in a process known as proteolysis. A number of different proteases are usually required to break down a protein completely into all its constituent amino acids.
Transamination A reaction involving the transfer of an amine group between two molecules, usually an amino acid and an α-keto acid.
KEY POINTS
■ Cheeses owe their smells and flavours to small volatile molecules. These are produced by enzymes breaking down three types of molecule present in milk: casein (a protein), lipid in milk fat and lactose (milk sugar).
■ Different enzymes in different cheeses produce different smelly molecules.
■ Blue cheeses owe their characteristic smell to certain alkan-2-ones.
■ The smell of Cheddar and other hard cheeses is a blend of the odours of several molecules, some of which by themselves would be objectionable.
ChemistryReviewExtras
Check your answers at www.hoddereducation.co.uk/chemistryreviewextras





