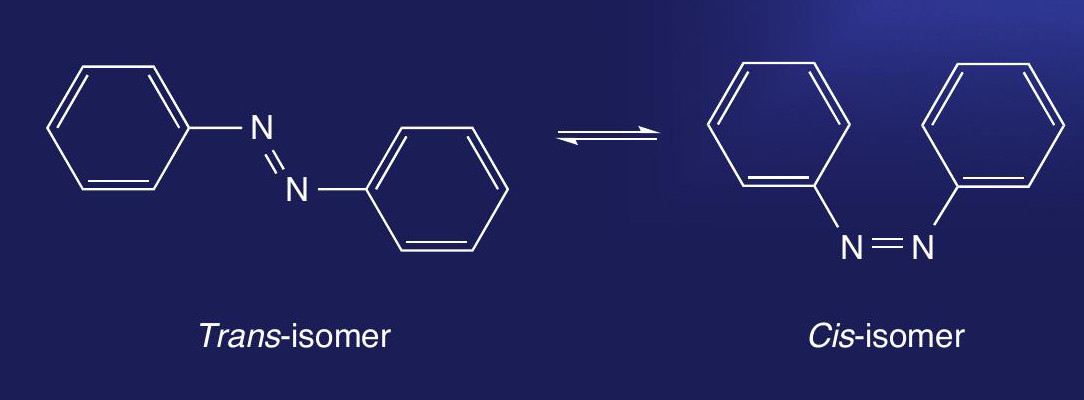
Molecules containing a diazo group (e.g. azobenzene) can exist as cis- and trans-isomers (Figure 1). Cis/ trans isomerism is a special case of E/Z isomerism (see CHEMISTRY REVIEW, Vol. 23, No. 2, p. 31). This is a form of stereoisomerism caused by restricted rotation about a double bond, when two different groups are attached to each atom at either end of the double bond.
In cis/trans isomerism at least one substituent group on the atoms at each end of the double bond is identical to the other. In the cis-isomer the identical groups are on the same side of the double bond. In the trans-isomer these groups are on opposite sides of the double bond.
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




