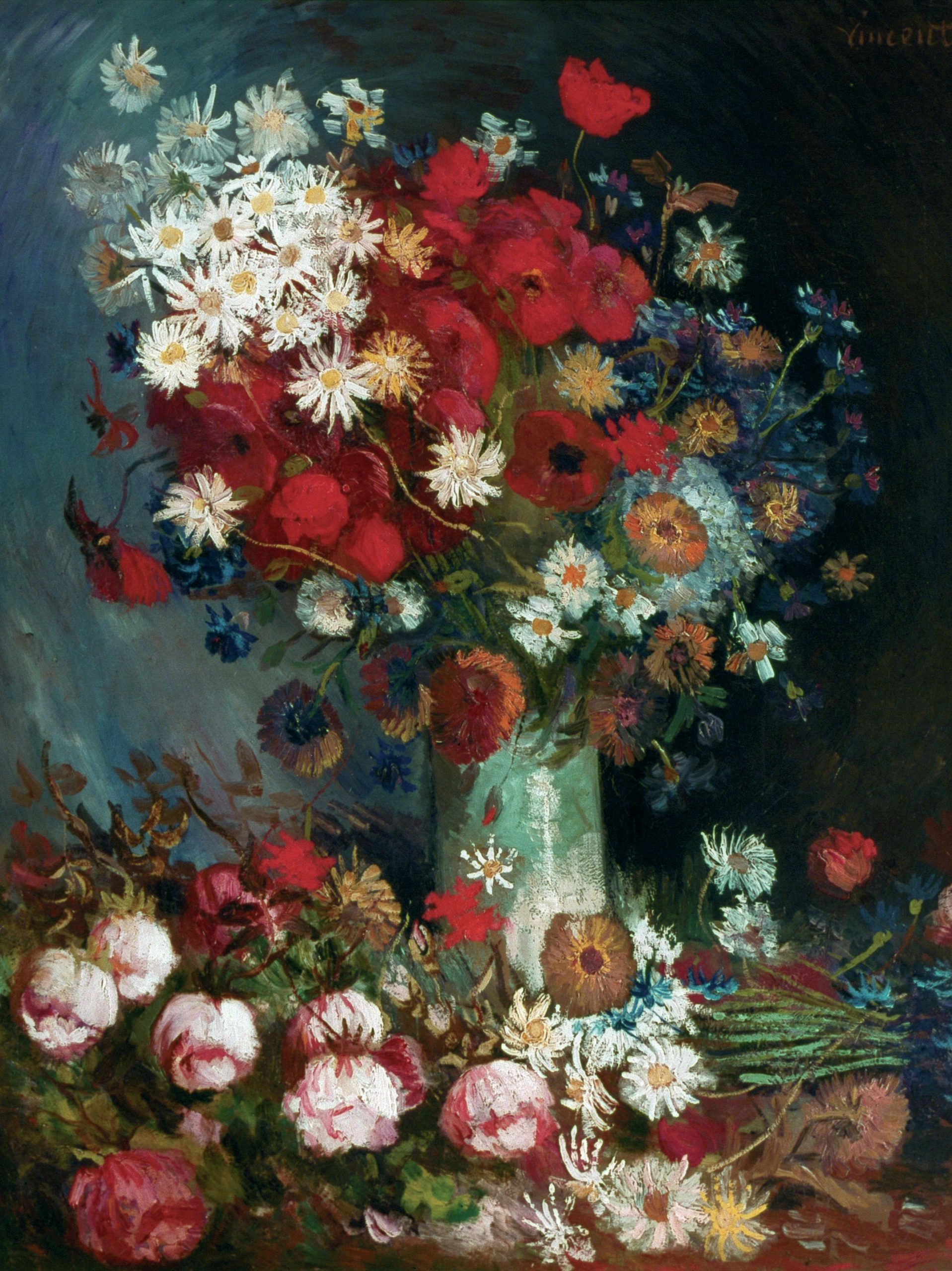
Spectroscopy has played its part in the rediscovery of lost works of art. In 2012, X-ray fluorescence spectroscopy was used to analyse a painting called Still Life with Meadow Flowers and Roses tentatively attributed to the world’s most popular artist, Vincent Van Gogh.
X-rays can knock out an electron from a low-energy orbital of an atom. An electron from a higher-energy orbital loses energy to fall into that orbital to replace it. The energy is released from the atom as a photon of light, with a wavelength corresponding to the energy gap between the electrons. This is specific to particular atoms, and so can be used to identify different elements. As X-rays are very penetrating, they can pass straight through a material like a canvas, and identify elements in different layers.
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




