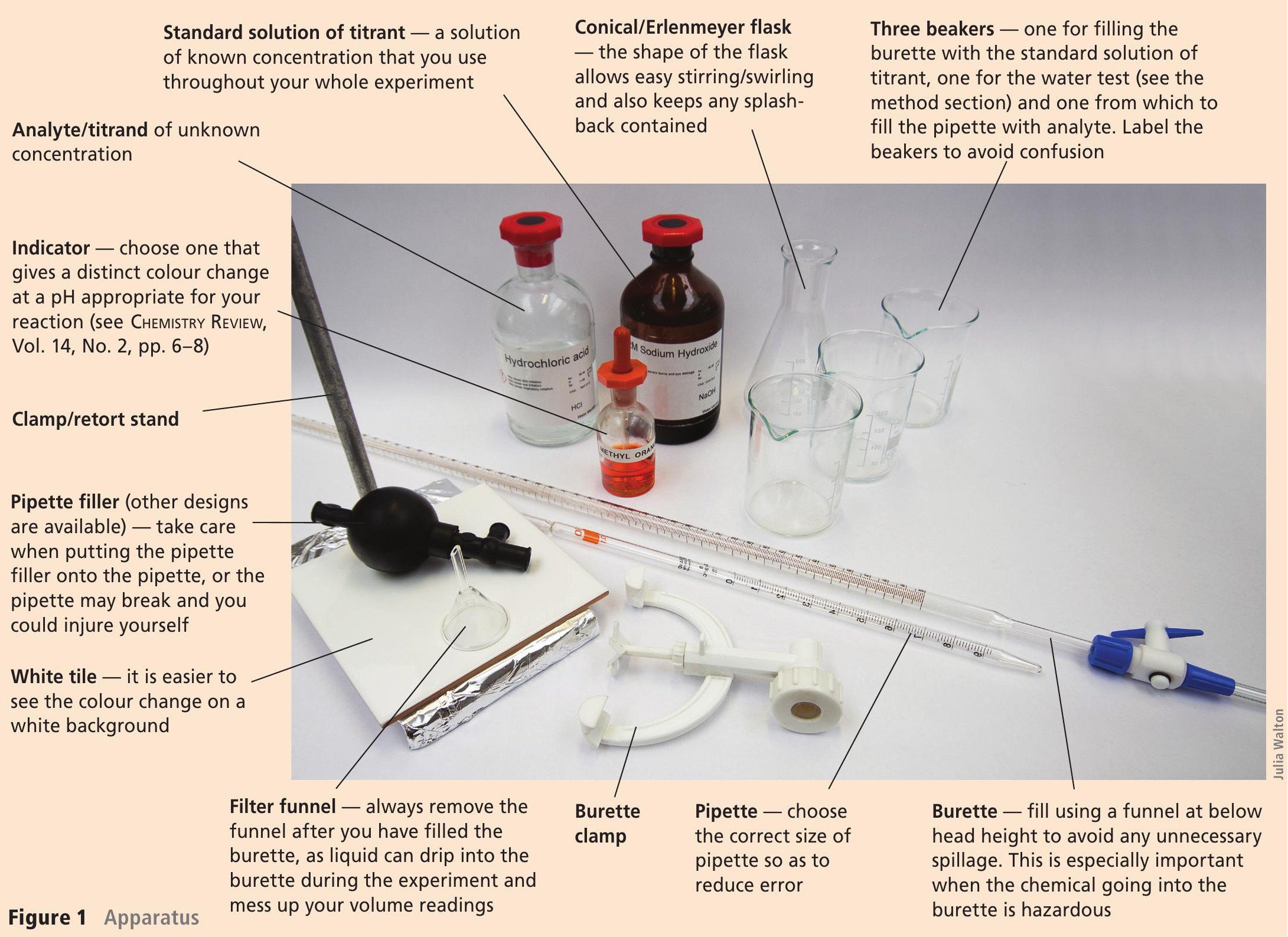
All exam specifications require you to be able to carry out a titration.
Best practice in the laboratory is handed down through generations of chemists. In this outline of how to perform a titration there are lots of little snippets of wisdom passed on to me by my teachers, along with some of my own tips. See Figure 1 for the apparatus you need.
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




