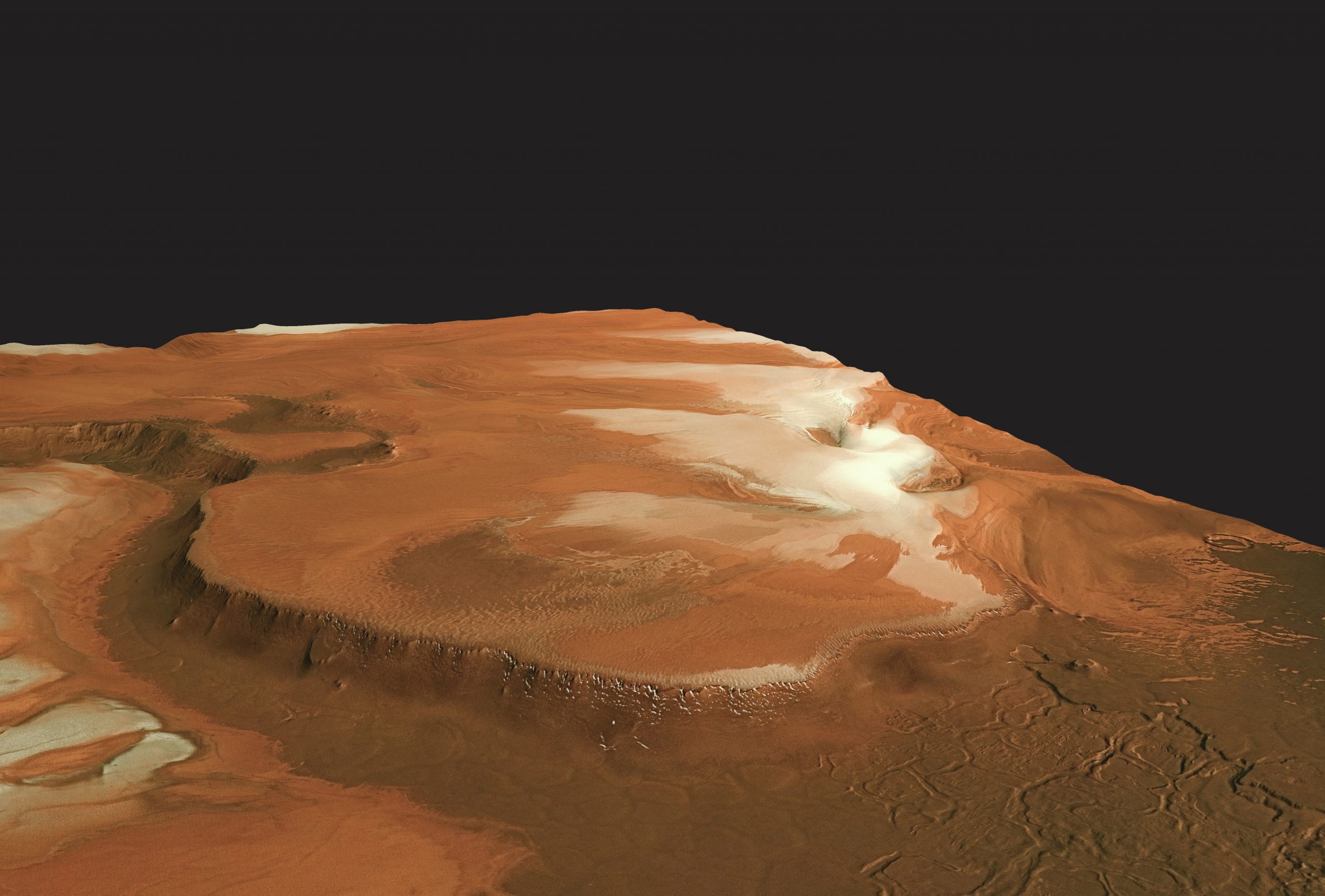
Mars, the red planet, is under close observation at the moment from the NASA Rovers (Figure 1 and marsrovers.nasa.gov/home/index.html) and the ESA Mars Express (Figure 2 and www.esa.int/esaMI/Mars_Express). Careful inspection of the poles reveals a white covering of what looks like snow — the polar ice caps (Figure 3).
What makes these different to the Earth’s poles is that the Martian atmosphere is nearly all carbon dioxide, although atmospheric pressure is about 100 times less than on Earth. Spectroscopy of the reflected light from the ‘snow’ reveals that the ice is in fact ‘dry ice’, or solid carbon dioxide. Just like on Earth, Mars has seasons, with the south pole experiencing a temperature of 125 K (–148ºC) in the winter but 225 K (–49ºC) in the summer, so the ice melts, or to be more accurate it sublimes. Unlike water ice, carbon dioxide does not melt from a solid to a liquid and then to a gas when heated, but instead (if the pressure is low enough) passes straight from the solid to the gas phase. The reason why carbon dioxide sublimes is that it has relatively weak forces between the molecules. Unlike water, where hydrogen bonds between the water molecules are comparatively strong, the forces between CO2 molecules are comparatively weak and even at low temperatures there is sufficient energy to make them evaporate (Figure 4).
Your organisation does not have access to this article.
Sign up today to give your students the edge they need to achieve their best grades with subject expertise
Subscribe




